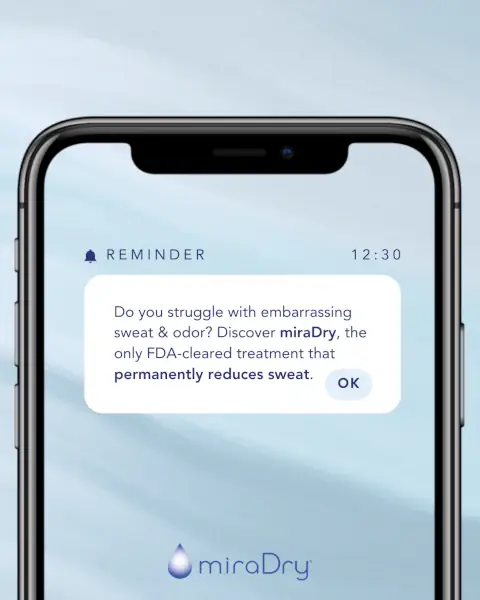
PSA- you don’t have to struggle with underarm sweat and odor! Put yourself first and invest in the ultimate self-love gift – permanent sweat reduction with miraDry! ✨
✨ Feel fresh. Feel clean. Feel like YOU again.
Visit our website now to learn more about our treatment!
Link in bio 🔗
#miraDry #SweatStopsHere #SweatSolutions #ALifeWithoutSweat #SweatyArmpits #SweatTreatment #Wellness #Selfcare #OdorSolutions